Following the Travis County Medical Society Town Hall Meeting, we would like to take this time to provide an update regarding the COVID-19 vaccine.
Two COVID-19 vaccines are expected to arrive in Texas around December 17-18, by two different manufacturers, Moderna and Pfizer/BioNTech. Both vaccines are similar in their active ingredient, messenger RNA, to allow the body to develop antibodies against the COVID-19 spike protein.
Key Assumptions for COVID-19 Vaccine:
- Limited doses may be available by mid December 2020, but supply will increase substantially in 2021
- Initial supply will either be approved as a licensed vaccine or authorized for use under an Emergency Use Authorization issued by the FDA
- Two doses, separated by 21 or 28 days, will be needed for immunity for most COVID-19 vaccines
- Risk of side effects are low and are similar to those of the influenza vaccine, such as local redness, swelling, soreness, and mild flu-like symptoms
The COVID-19 vaccine timeline will follow 4 phases based on the activities allowed by availability of supplies:
- Phase 1 (November-December 2020)
- Limited doses
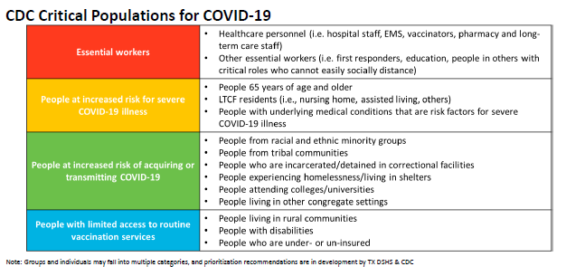
- Priority groups based on CDC guidance when sufficient vaccine is made available: critical infrastructure workers, individuals who are at high risk for severe COVID disease, individuals who are at high risk for acquiring or transmitting COVID, people with limited access to vaccines (see below for CDC Critical Populations for COVID-19)
- Phase 2 (January-July 2021)
- Number of doses available increases (~660M nationwide)
- Our practice will likely fall in this phase, and we can expect to have the vaccines available in our office sometime in early 2021
- Phase 3 and Phase 4
- Likely excess supply
- Outreach to hard-to-reach populations
- Plan for potential seasonal vaccine
We will email our patients when our practice is able to administer the COVID-19 vaccine. Please visit our blog and check your emails regularly for ongoing updates regarding the COVID-19 vaccine.